Bone elasticity
Cortical bone of human adults is the focus of this webpage. Cortical bone, which is the dense outer shell of bones (level a on figure), is also called 'osteonal' bone because it is made of secondary osteons, a product of the remodeling process (10% of the skeleton may be replaced each year).
Cortical bone hierarchical levels
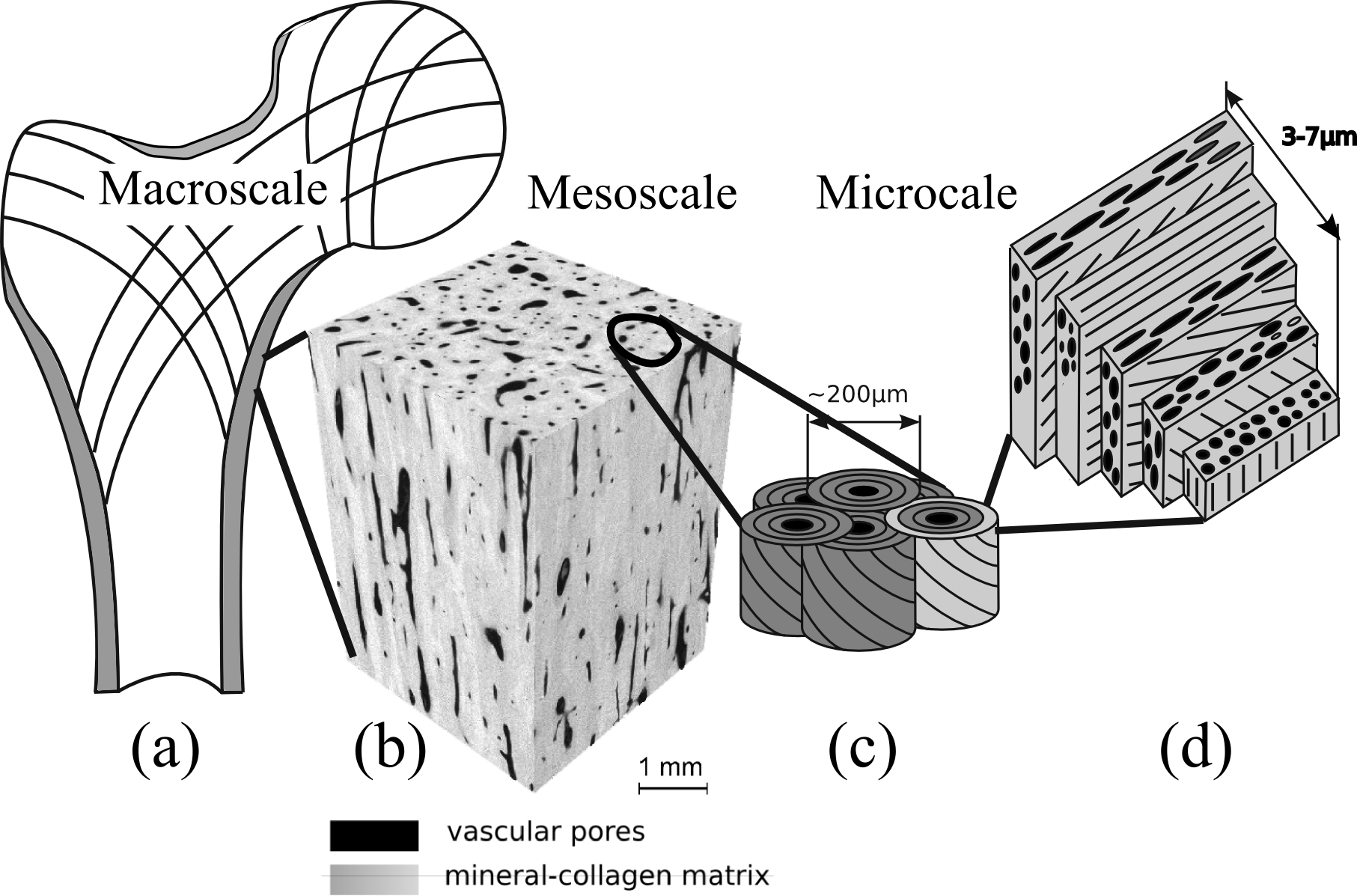
Hierarchical cortical bone structure. After Rho et al, 1998 Med Eng. Phys 20:92 and Reisinger et al. 2010 Biomech Model Mechanobiol 9:499
Cortical bone is a network of mineralized collagen fibrils pervaded by pores which fall in two categories. The lacuno-canalicullar network (LCN) encloses the osteocytes cells in lacunae (~10 mm) and their processes in the canaliculi. The vascular network (level b on figure), which encloses vessels and nerves, consists of the Haversian channels (~50 mm diameter) and Volkman's canals. Mineralized collagen fibrils are organized in concentric layers around Haversian channels forming osteons (level c on figure). Each osteon is the result of a cycle of resorption and formation of bone by a group of cells (basic multicellular unit, BMU). Collagen fibrils are oriented at angles with respect to the Haversian channel (level d on figure) according to a complex pattern which is not thoroughly understood but is supposed to be influenced by the orientation and type of macroscopic loads on the bone. The vascular porosity can be imaged in 3D with high-resolution CT (in vitro), allowing a separation of the vascular pores from the rest of the tissue.
Variability and anisotropy of cortical bone elasticity
The elasticity of cortical bone is characterized by at least five moduli conveniently collected in a mathematical object called the stiffness tensor. Important is to note that the knowledge of all these constants is required in order to calculate strains in bones for arbitrary load type and direction. Large relative variations of the tissue elastic moduli, typically of the order of 100%, are observed across anatomical locations and between subjects due to variations in density and preferential orientation of collagen fibers. It is long established that elasticity depends on apparent bone density, which is a combination of the mineral content of the extracellular matrix and of the vascular porosity. For the young human adult, comparable elastic properties were reported for different sites (tibia and femur shaft, etc.). This is not anymore the case in the older adult when the vascular porosity may vary typically between 5 and 25% due to imbalance of the remodeling, introducing large inter-site and inter-individual elastic variations. In addition, anisotropy ratios typically vary between 1.5 and 2.5 (see Figure), which means that depending on the orientation of the microstructure with respect to the direction of an external loading, strains in bone may be in proportion of 1.5 up to 2.5.
The anisotropy of cortical tissue is in part due to the preferential orientation of the vascular pores but to a larger extent to the anisotropy of the extracellular matrix caused by the preferential orientation of collagen fibers (see this paper).
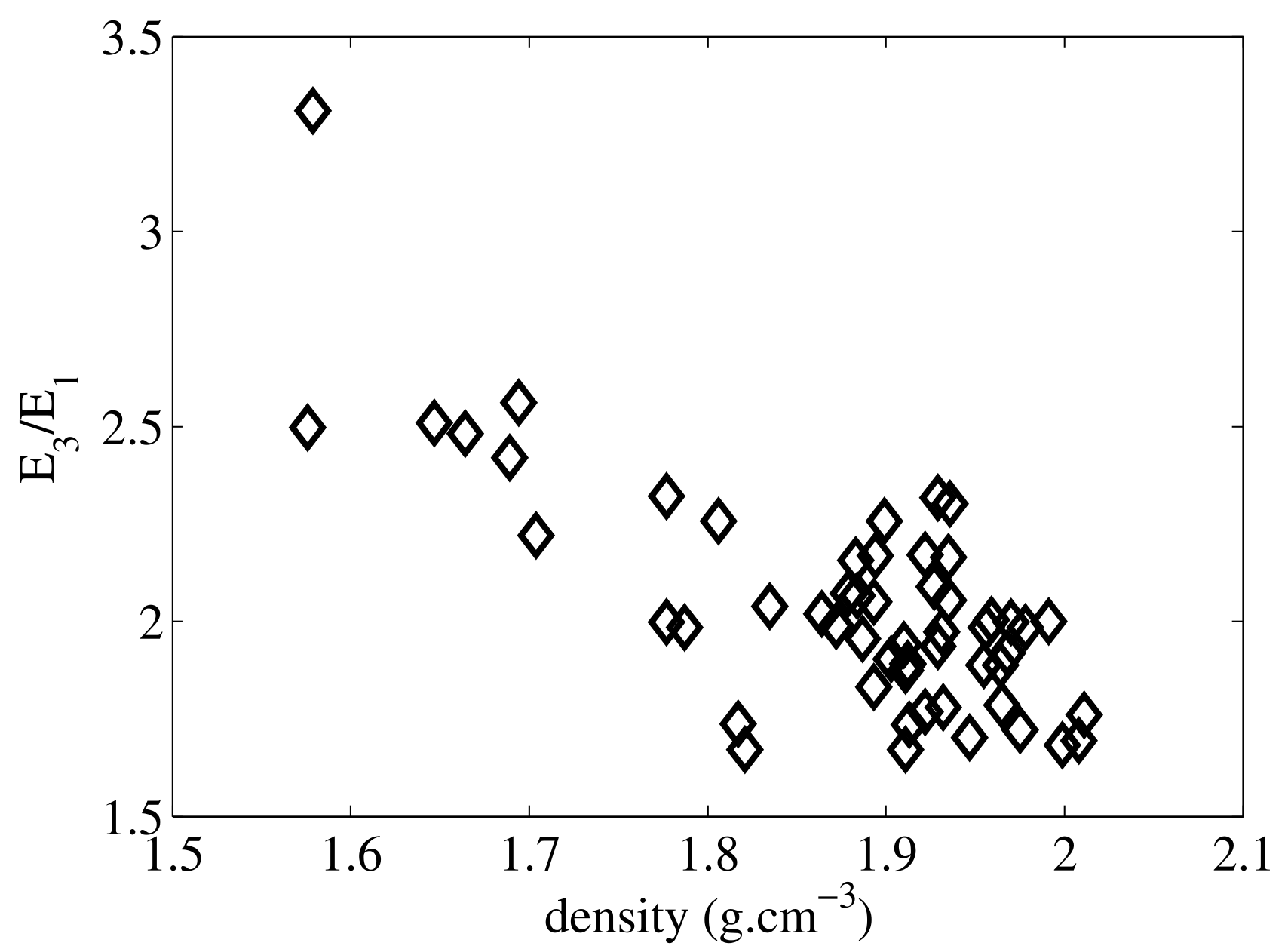
Young's modulus anisotropy ratio as a function of mass density for a collection of 55 specimens from the mid-tibia diaphysis. Elasticity was measured with RUS
Modeling of mesoscale elastic properties
At the so-called mesoscale, that is to say at a scale of a volume of approximately 1mm3, cortical bone can be regarded as a homogenized material with anisotropic properties. Mesoscale properties play a central role in bone biomechanics. On the one hand, in a finite element model of the whole bone, the elastic properties are typically represented with homogeneous properties at the mesoscale (in many approaches using QCT, this corresponds grossly to the millimeter-sized voxel). On the other hand, the mesoscale properties depend on the structure and composition of the tissue at the smaller hierarchical levels. We have shown that all mesoscale cortical bone elastic moduli (shear and longitudinal elastic coefficients in different direction with respect to the bone axis) strongly depend on vascular porosity in the elderly (see Figure). To some extent, the vascular porosity can be modeled as cylindrical voids filled with a water-like fluid and the extracellular matrix by a homogeneous transverse isotropic material. The homogenized properties of such a material can be calculated in the framework of continuum mechanics. On the Figure we compare measured and theoretical mesoscale elastic coefficients for a typical range of porosity. Theoretical values were calculated with the asymptotic homogenization method BonHom program .
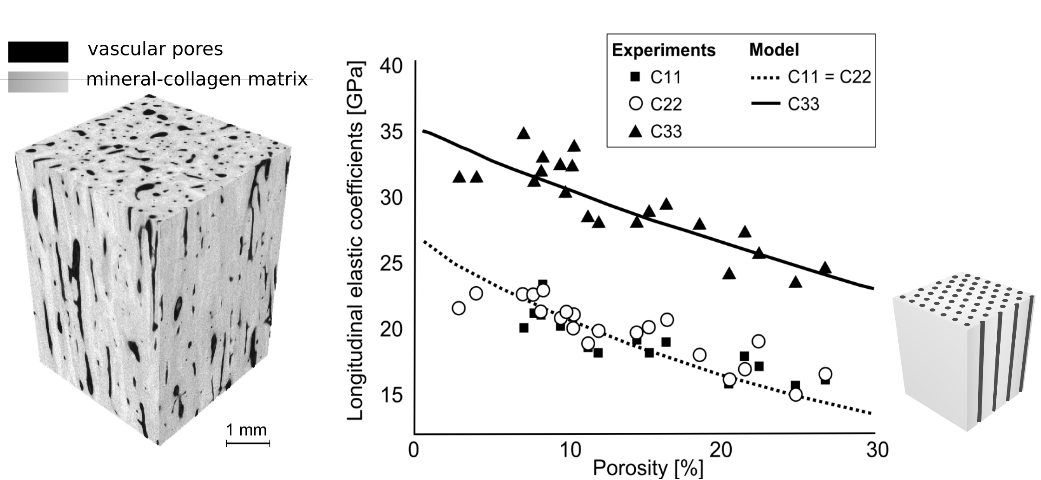
Left: CT image of human mid-femur diaphysis specimen. Right: comparison of measured stiffness coefficients and calculated coefficients calculated with homogenization (Granke et al, Bone 2011)
Research questions
Cortical bone elastic properties may vary depending on the bone considered (i.e., tibia, vs femur or skull), on the anatomical site in a bone (i.e., anterior vs. posterior site), and on the individual (age, pathology, etc.). It is important to dispose of methods to assess bone elasticity with sufficient precision to assess these variations.
Conventional mechanical testing hardly meets the requirements for complete and accurate bone elasticity measurement. This is due to the anisotropy of bone (mechanical testing does not allow the measurement of all elastic coefficients on one specimen) and to the small size of the specimens dictated by the small thickness of cortices in human bone. The most adapted methods to measure bone elasticity use ultrasound waves. Ultrasound methods, being non destructive, are adapted to measure several elastic coefficients on a single specimen. The classical method developed in the 1970's, which deduces elasticity from ultrasound velocities of shear and longitudinal waves, has been extensively used to study bone elastic anisotropy. Resonant Ultrasound Spectroscopy (RUS) is emerging as an alternative to the latter method to measure more accurately the stiffness tensor. RUS is also advantageous to measure particularly small bone specimens (~1mm).
Two research areas for which measuring elastic properties is an issue are shortly described below:
- Cortical tissue elasticity, being related to porosity and the quality of the extracellular mineralized matrix, could be a marker of bone pathologies. Documentation and modeling of elastic properties is an important issue related to the in vivo assessement of bone elastic properties with ultrasound techniques.
- Ascertaining cortical tissue elasticity is a key aspect of the research on bone remodeling. Indeed, the strain in the tissue or the strain energy density, two quantities often considered to trigger remodeling, are function of both loading (local stress on tissue) and elastic properties.
Related publications of our group
- Rohrbach D, Grimal Q, Varga P, Peyrin F, Langer M, Laugier P, Raum K. Distribution of mesoscale elastic properties and mass density in the human femoral shaft. Connect Tissue Res. 2015 Apr;56(2):120-32. Epub 2015 Mar 4.
- Granke M, Grimal Q, Parnell WJ, Raum K, Gerisch A, Peyrin F, Saied A, Laugier P, To what extent can cortical bone millimeter-scale elasticity be predicted by a two-phase composite model with variable porosity?, Acta Biomater, 2015, 12 , pp. 207-15
- Parnell WJ, Vu MB, Grimal Q, Naili S, Analytical methods to determine the effective mesoscopic and macroscopic elastic properties of cortical bone, Biomech Model Mechanobiol, 2012, 11 (6) , pp. 883-901
- Rohrbach D, Lakshmanan S, Peyrin F, Langer M, Gerisch A, Grimal Q, Laugier P, Raum K, Spatial distribution of tissue level properties in a human femoral cortical bone, J Biomech, 2012, 45 (13) , pp. 2264-70
- Granke M, Grimal Q, Saied A, Nauleau P, Peyrin F, Laugier P, Change in porosity is the major determinant of the variation of cortical bone elasticity at the millimeter scale in aged women, Bone, 2011, 49 (5) , pp. 1020-6
- Grimal Q, Raum K, Gerisch A, Laugier P, A determination of the minimum sizes of representative volume elements for the prediction of cortical bone elastic properties, Biomech Model Mechanobiol, 2011, 10 (6) , pp. 925-37
- Grimal Q, Rus G, Parnell WJ, Laugier P, A two-parameter model of the effective elastic tensor for cortical bone, J Biomech, 2011, 44 (8) , pp. 1621-5
- Parnell WJ, Grimal Q, The influence of mesoscale porosity on cortical bone anisotropy. Investigations via asymptotic homogenization, J R Soc Interface, 2009, 6 (30) , pp. 97-109
- Grimal Q, Raum K, Gerisch A, Laugier P, Derivation of the mesoscopic elasticity tensor of cortical bone from quantitative impedance images at the micron scale, COMPUTER METHODS IN BIOMECHANICS AND BIOMEDICAL ENGINEERING, 2008, 11 (2) , pp. 147-157